-base.png?x=856&format=.jpg)
Innovative implants are undergoin highly diverse and strongly cooperative development processes. A wide variety of industries with a large number of players are involved in the successful development of an implant system. In addition to the technological requirements, the problems of global supply chains, medical device regulation and practical use in everyday clinical practice must be considered as a whole.
In addition to the latest technological developments, voices from the field of practical medical application as well as from the field of medical device regulation will have their say and present their diverse demands on the development process. Be there, learn about the latest innovations in the field of high-tech implants and discuss the future of the industry with experts. There is an opportunity to register at discounted early bird rates until February 10.
The program and registration option can be found at ivam.com/events/innovative_implant_technologies
Contact: Dr. Victoria Jakobi, IVAM
vj@ivam.de
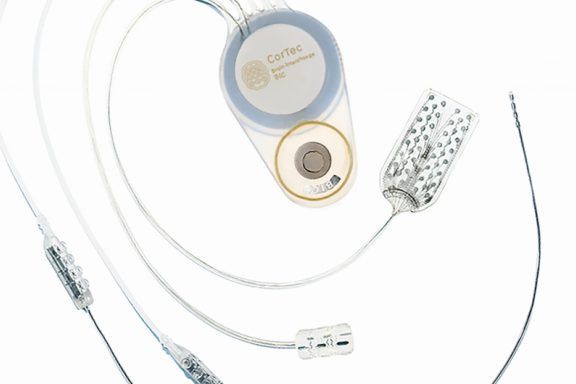
Freiburg-based CorTec GmbH and Heraeus Medevio, headquartered in Minneapolis, USA, have entered into a strategic partnership in the field of …
Autonomous systems, robotics and high-tech components take center stage: At XPONENTIAL Europe 2026 in March, IVAM will for the first …
CorTec has completed the second successful implantation of its fully implantable brain-computer interface system as part of an FDA-approved clinical …